EntrolyticsMD, the world's first imaging platform for digestive diseases has been listed with the FDA. 40 Million people are affected by digestive diseases (including inflammatory bowel disease and GI cancers) costing $119.6 billion in 2018. EntrolyticsMD is already in use across over 300 investigator projects, for AI deployment and in clinical trials.
Here's your TLDR of the announcement ⏱ ⬇️
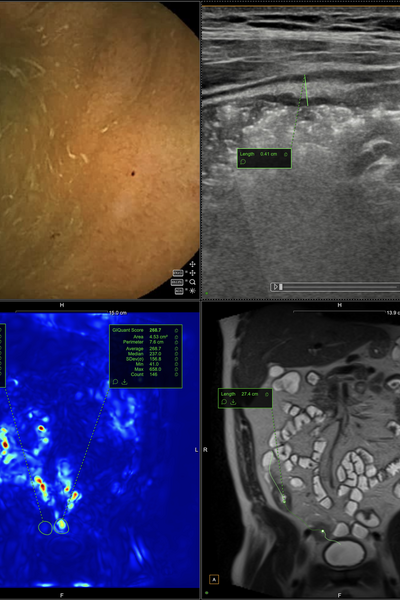
What is Entrolytics? A Web-based 'PACS' designed for the assessment and management of digestive diseases.
What's wrong with regular PACS? They have to support a wide range of clinical domains #neuro , #msk , #oncology etc (a 'horizontal' approach) Entrolytics optimises features for #digestivediseases (a 'vertical' approach).
How many people suffer from digestive diseases? Around 400M people.
This includes #crohnsdisease , #perianaldisease , #ibd subtypes and #cancer
EntrolyticsMD has already been used in over 300 #research projects, and #clinicaltrials / multicenter trials globally.
Existing multicenter trials include FIBROTARGET and CAMEO.
Motilent’s EntrolyticsMD is now ready for clinical use in the United States.
- EntrolyticsMD, the world's first imaging platform for digestive diseases has been listed with the FDA.
- 40 Million people are affected by digestive diseases (including inflammatory bowel disease & GI cancers) costing $119.6 billion in 2018.
- EntrolyticsMD is already in use across over 300 investigator projects, for AI deployment and in clinical trials.
London, UK - 20/11/23 - Motilent announces that EntrolyticsMD, the world's first imaging platform designed for the assessment of digestive disease has been listed with the FDA. The cutting-edge medical imaging platform is optimised for MRI, CT, Intestinal Ultrasound as well as other complementary modalities including video endoscopy.
Globally, over 400 million [1] people are affected by digestive diseases, with 40 million in the United States alone. This results in millions of annual clinical visits with the healthcare expenditures totalling $119.6 billion in 2018. [2]
EntrolyticsMD integrates streamlined data management and advanced processing tools on a secure collaborative web-based platform. This includes the latest AI-powered tools and Motilent’s existing FDA Cleared product GIQuant. EntrolyticsMD has already been used in over 300 research projects, and clinical and multicenter trials globally. Existing multicenter trials include CAMEO & FIBROTARGET.
Alex Menys (CEO) says: “Healthcare continues to get more complex and expensive. Nowhere is this more true than in digestive disease with optimal healthcare demanding input from multiple specialties. Gastroenterologists, surgeons, radiologists, specialist nursing, nutrition and many more. A key challenge lies in how we aggregate and interpret data in areas like IBD (Inflammatory Bowel Disease) where diagnosis and management is complex and evolving. EntrolyticsMD is our latest contribution to this field, a powerful platform that now brings together multi-modality data to guide research, support trials and help patients by bringing often siloed data together, in the clinic. This regulatory milestone represents Motilent’s commitment to quality, our intent to tackle the tough challenges and is a key step forward in our mission to change the way we see the gut.
Dr. Jonathan Dillman (Cincinnati Children's Hospital) says - “In the past year I’ve moved many of my GI research projects onto EntrolyticsMD. The platform is easy to use, facilitates both multi-reader and multi-centre research, and handles all types of imaging, including endoscopy! EntrolyticsMD enables high-quality research, allows rapid image assessment (including numerous GI-specific tools), and has the potential to not only transform research but also bring novel image analysis methods to the clinic.”
This FDA listing represents a key milestone in the development of patient management in digestive disease and is a major advancement in Motilent's mission to change the way we see the gut.
For more information, please contact:
Beth Fisher
Motilent
Beth.fisher@motilent.io
Motilent.io
About Motilent: Motilent is at the forefront of innovative medical imaging and collaborative technology, helping to improve the lives of patients with digestive diseases.
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10088561/
[2]https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10088561/#:~:text=In%20the%20United%20States%2C%20digestive,billion%20in%202018%20(1).